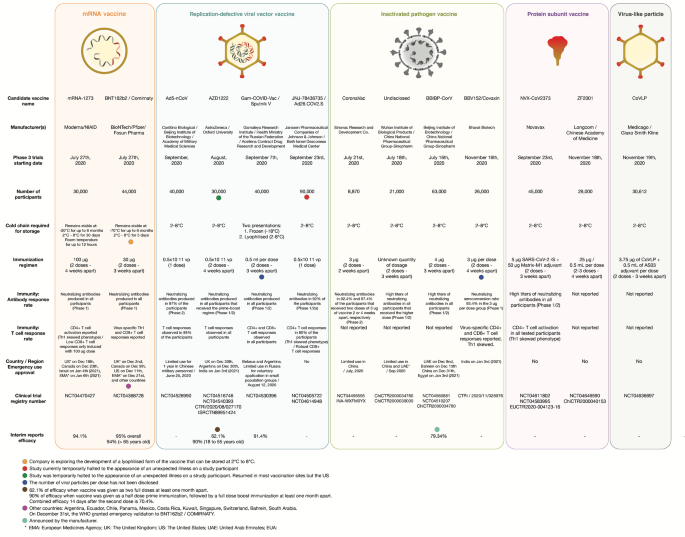
Vaccine Efficacy Rate Comparison Sinovac : How Does Sinovac S Efficacy Rate Compare To Other Covid 19 Vaccines The China Africa Project _ All other vaccines without available data:
Vaccine Efficacy Rate Comparison Sinovac : How Does Sinovac S Efficacy Rate Compare To Other Covid 19 Vaccines The China Africa Project _ All other vaccines without available data:. Effectiveness has also been shown in an observational study in sao paulo in the presence of p1 circulation (83% of samples). Jun 16, 2021 · sinopharm's other vaccine, developed via a wuhan unit and not listed for emergency use by the who, has an efficacy rate of around 73%. Authorized for use in the european union. In early april, the cdc and fda issued a joint recommendationfor states to halt use of the johnson & johnson vaccine "out of an abundance of caution" during an investigation into reports of six rare, but serious clotting problems among women ages 18 to 48, occurring six to 13 days after vaccination (the recommendation fell short of an order to stop using the vaccine, leaving final decisions to the individual states). This clears the way for states to resume vaccinations with the johnson & johnson vaccine.
Moderna's vaccine was the second one authorized for emergency use in the u.s.—it received fda eua on december 18, 2020, about a week after the pfizer vaccine. Jul 26, 2021 · sinovac efficacy and effectiveness in the real world. All other vaccines without available data: Status:emergency use in the u.s.; See full list on yalemedicine.org
This clears the way for states to resume vaccinations with the johnson & johnson vaccine.
Feb 25, 2021 · the clinical trial in turkey, however, produced an efficacy rate of 91.25 per cent. Authorized for use in the european union (under the name comirnaty). Effectiveness has also been shown in an observational study in sao paulo in the presence of p1 circulation (83% of samples). All other vaccines without available data: Jan 12, 2021 · overall efficacy of sinovac vaccine in brazil just above 50% on tuesday, butantan explained the 78% was calculated considering the mild, moderate and severe cases, officials said. In indonesia, a local trial demonstrated an efficacy rate of 65%, but the trial had only 1,620 participants. Status:emergency use in the u.s.; Dosage:two shots, 28 days apart common side effects: But on friday, april 23, the food and drug administration (fda) ended its recommended pause on the vaccine and will add a warning label about an uncommon, but potentially serious, blood clotting disorder. The company's clinical trials are demonstrating dramatically varying efficacy rates. Recommended for: anyone 12 or older. See full list on yalemedicine.org Similar to the pfizer vaccine, side effects can include chills, headache, pain, tiredness, and/or redness and swelling at the injection site, all of which generally resolv.
Data on sinovac's coronavac vaccine is limited since a number of international studies on the vaccine are. Approval would make the vaccine easier to distribute. May 14, 2021 · astrazeneca used these two percentages to average an effectiveness rate of 76 percent. In indonesia, a local trial demonstrated an efficacy rate of 65%, but the trial had only 1,620 participants. Chills, headache, pain, tiredness, and/or redness and swelling at the injection site, all of which generally resolve within.
This decision followed a vote by a panel of advisers to the centers for disease control and prevention (cdc) to end the pause.
In early april, the cdc and fda issued a joint recommendationfor states to halt use of the johnson & johnson vaccine "out of an abundance of caution" during an investigation into reports of six rare, but serious clotting problems among women ages 18 to 48, occurring six to 13 days after vaccination (the recommendation fell short of an order to stop using the vaccine, leaving final decisions to the individual states). Dosage:two shots, 28 days apart common side effects: There are two key differences: This decision followed a vote by a panel of advisers to the centers for disease control and prevention (cdc) to end the pause. In indonesia, a local trial demonstrated an efficacy rate of 65%, but the trial had only 1,620 participants. See full list on yalemedicine.org Moderna's vaccine was the second one authorized for emergency use in the u.s.—it received fda eua on december 18, 2020, about a week after the pfizer vaccine. Effectiveness has also been shown in an observational study in sao paulo in the presence of p1 circulation (83% of samples). All other vaccines without available data: Similar to the pfizer vaccine, side effects can include chills, headache, pain, tiredness, and/or redness and swelling at the injection site, all of which generally resolv. Approval would make the vaccine easier to distribute. This clears the way for states to resume vaccinations with the johnson & johnson vaccine. But on friday, april 23, the food and drug administration (fda) ended its recommended pause on the vaccine and will add a warning label about an uncommon, but potentially serious, blood clotting disorder.
Approval would make the vaccine easier to distribute. Recommended for: anyone 12 or older. Also, the moderna vaccine was slightly less effective in clinical trials—about 86%—in people who are 65 and older. May 14, 2021 · astrazeneca used these two percentages to average an effectiveness rate of 76 percent. Jan 12, 2021 · overall efficacy of sinovac vaccine in brazil just above 50% on tuesday, butantan explained the 78% was calculated considering the mild, moderate and severe cases, officials said.

Feb 25, 2021 · the clinical trial in turkey, however, produced an efficacy rate of 91.25 per cent.
Jan 12, 2021 · overall efficacy of sinovac vaccine in brazil just above 50% on tuesday, butantan explained the 78% was calculated considering the mild, moderate and severe cases, officials said. Status:emergency use in the u.s.; Jul 26, 2021 · sinovac efficacy and effectiveness in the real world. Data from a brazilian clinical trial pegged coronavac's effectiveness at 50.4 per cent. Two shots, 21 days apart common side effects: We find the ratio of the efficacy between b.1.351 and b.1.1.7 as observed in the qatar study. Jun 16, 2021 · sinopharm's other vaccine, developed via a wuhan unit and not listed for emergency use by the who, has an efficacy rate of around 73%. Also, the moderna vaccine was slightly less effective in clinical trials—about 86%—in people who are 65 and older. Data on sinovac's coronavac vaccine is limited since a number of international studies on the vaccine are. See full list on yalemedicine.org On february 27, 2021, the fda granted emergency use approval for a different type of vaccine, called a carrier, or virus vector, vaccine. Feb 25, 2021 · the clinical trial in turkey, however, produced an efficacy rate of 91.25 per cent. In early april, the cdc and fda issued a joint recommendationfor states to halt use of the johnson & johnson vaccine "out of an abundance of caution" during an investigation into reports of six rare, but serious clotting problems among women ages 18 to 48, occurring six to 13 days after vaccination (the recommendation fell short of an order to stop using the vaccine, leaving final decisions to the individual states).
Jan 12, 2021 · overall efficacy of sinovac vaccine in brazil just above 50% on tuesday, butantan explained the 78% was calculated considering the mild, moderate and severe cases, officials said sinovac vaccine efficacy. Also, the moderna vaccine was slightly less effective in clinical trials—about 86%—in people who are 65 and older.